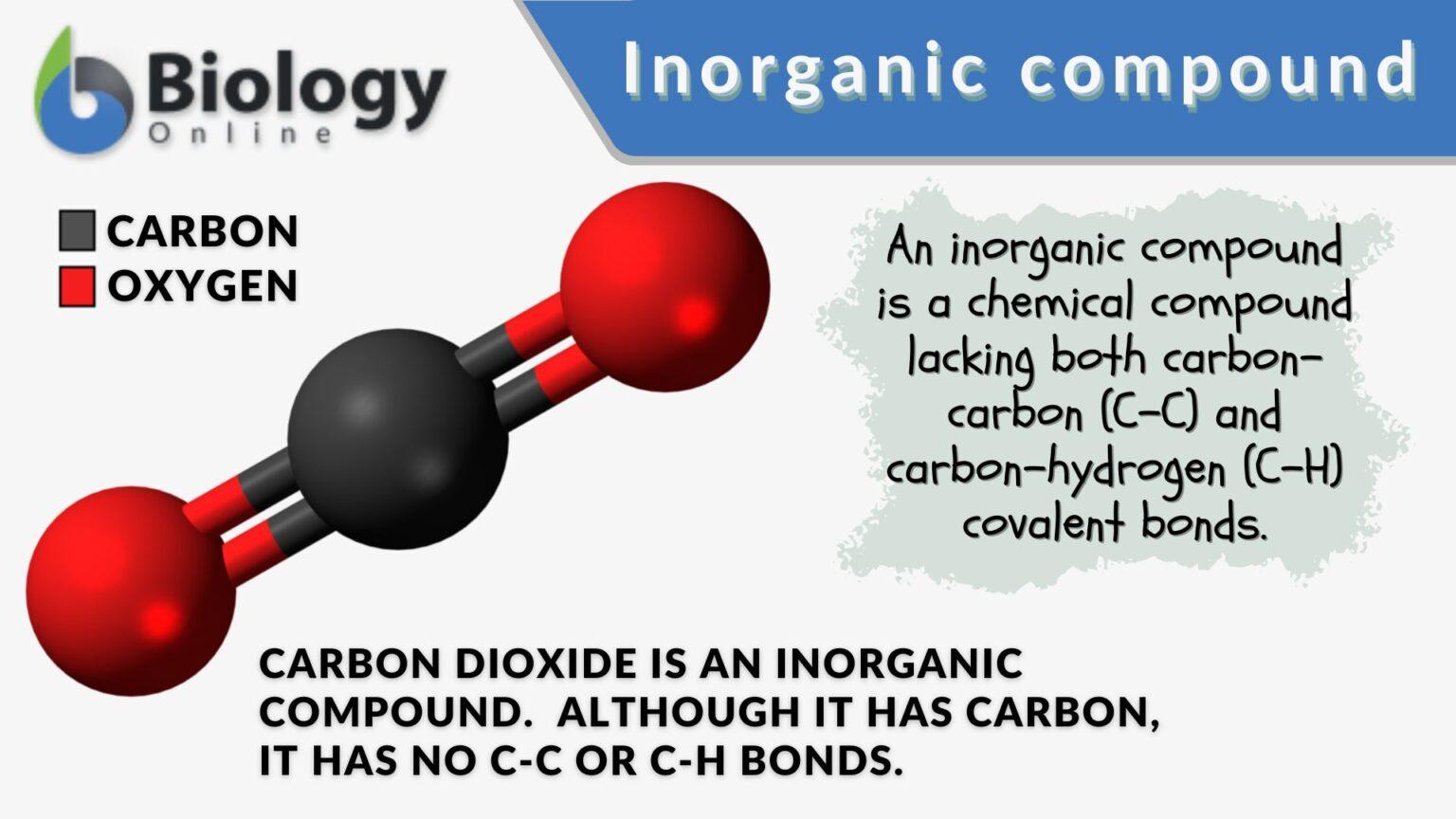
Inorganic Base Name . A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. Many bases, like soaps, are slippery to the touch. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Aqueous solutions of bases are also electrolytes. Bases often have a bitter taste and are found in foods less frequently than acids. Bases can be either strong or weak, just as acids can. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Bases have properties that mostly contrast with those of acids. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Bases also change the color of. Most bases are minerals that react with acids.
from www.biologyonline.com
A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. Most bases are minerals that react with acids. Bases can be either strong or weak, just as acids can. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Bases also change the color of. Aqueous solutions of bases are also electrolytes. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Bases often have a bitter taste and are found in foods less frequently than acids. Many bases, like soaps, are slippery to the touch. Bases have properties that mostly contrast with those of acids.
compound Definition and Examples Biology Online Dictionary
Inorganic Base Name Most bases are minerals that react with acids. Bases have properties that mostly contrast with those of acids. Bases also change the color of. Bases can be either strong or weak, just as acids can. Many bases, like soaps, are slippery to the touch. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Aqueous solutions of bases are also electrolytes. Most bases are minerals that react with acids. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Bases often have a bitter taste and are found in foods less frequently than acids.
From www.slideserve.com
PPT Naming Acids and Bases PowerPoint Presentation, free download Inorganic Base Name Bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods less frequently than acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and. Inorganic Base Name.
From www.pathwaystochemistry.com
Nomenclature of Acids Pathways to Chemistry Inorganic Base Name Many bases, like soaps, are slippery to the touch. Bases often have a bitter taste and are found in foods less frequently than acids. Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just as acids can. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds. Inorganic Base Name.
From www.slideserve.com
PPT Naming Acids and Bases PowerPoint Presentation, free download Inorganic Base Name this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods less frequently than acids. Bases can be either. Inorganic Base Name.
From www.flinnsci.ca
Ion Names, Formulas and Charges Chart Flinn Scientific Inorganic Base Name Bases can be either strong or weak, just as acids can. Many bases, like soaps, are slippery to the touch. a base is a substance that can neutralize the acid by reacting with hydrogen ions. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Bases also change the color. Inorganic Base Name.
From www.compoundchem.com
Compound Interest Pigment Compounds The Chemistry of Paint Inorganic Base Name Bases also change the color of. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Bases have properties that mostly contrast with those of acids. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Most bases are minerals that react with acids. Many. Inorganic Base Name.
From www.biologyonline.com
compound Definition and Examples Biology Online Dictionary Inorganic Base Name Bases often have a bitter taste and are found in foods less frequently than acids. Aqueous solutions of bases are also electrolytes. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Bases can be either strong or weak, just as acids can. Many bases, like soaps, are slippery to the. Inorganic Base Name.
From www.slideserve.com
PPT Naming Acids and Bases PowerPoint Presentation, free download Inorganic Base Name Bases also change the color of. Bases can be either strong or weak, just as acids can. Bases have properties that mostly contrast with those of acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic. Inorganic Base Name.
From www.slideserve.com
PPT ACIDS , BASES and SALTS PowerPoint Presentation, free download Inorganic Base Name Bases have properties that mostly contrast with those of acids. Bases often have a bitter taste and are found in foods less frequently than acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Aqueous solutions of bases are also electrolytes. this and the following section describe the rules for naming simple. Inorganic Base Name.
From scienceready.com.au
HSC Chemistry Nomenclature of Acids Science Ready Inorganic Base Name Many bases, like soaps, are slippery to the touch. this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). Bases. Inorganic Base Name.
From learningschoolmiszegzy.z22.web.core.windows.net
Writing Formulas From Names Inorganic Base Name a base is a substance that can neutralize the acid by reacting with hydrogen ions. A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. Bases often have a bitter taste and are found in foods less frequently than acids. Bases also change the color of. when a base. Inorganic Base Name.
From www.mindomo.com
Acids,Bases and Salts Mind Map Inorganic Base Name a base is a substance that can neutralize the acid by reacting with hydrogen ions. Many bases, like soaps, are slippery to the touch. A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. Most bases are minerals that react with acids. Bases also change the color of. this. Inorganic Base Name.
From www.teachoo.com
Classification of Acids on Basis of source, Concentration Teachoo Inorganic Base Name Bases often have a bitter taste and are found in foods less frequently than acids. A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. Bases have properties that mostly contrast with those of acids. Most bases are minerals that react with acids. Aqueous solutions of bases are also electrolytes. Many. Inorganic Base Name.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Inorganic Base Name Bases also change the color of. Bases can be either strong or weak, just as acids can. Bases have properties that mostly contrast with those of acids. Most bases are minerals that react with acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. A large class of inorganic compounds with the ability. Inorganic Base Name.
From chemistry.com.pk
Compounds As Pigments in Paints [Infographic] Inorganic Base Name Bases have properties that mostly contrast with those of acids. Bases can be either strong or weak, just as acids can. Aqueous solutions of bases are also electrolytes. A large class of inorganic compounds with the ability to react with, that is, neutralize acids to form salts. this and the following section describe the rules for naming simple covalent. Inorganic Base Name.
From www.bartleby.com
Bronsted Lowry Base In Chemistry bartleby Inorganic Base Name Bases can be either strong or weak, just as acids can. Bases also change the color of. Aqueous solutions of bases are also electrolytes. a base is a substance that can neutralize the acid by reacting with hydrogen ions. when a base reacts with any of the acids we have discussed, it accepts a proton (h +). . Inorganic Base Name.
From pressbooks.bccampus.ca
8.4 Relative Strengths of Acids and Bases Chemistry for Inorganic Base Name this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Bases have properties that mostly contrast with those of acids. Bases can be either strong or weak, just as acids can. A large class of inorganic compounds with the. Inorganic Base Name.
From 2bardesign.blogspot.com
Examples Of Bases 2bardesign Inorganic Base Name this and the following section describe the rules for naming simple covalent compounds, beginning with inorganic compounds and then turning to simple organic compounds that contain only carbon and hydrogen. Aqueous solutions of bases are also electrolytes. Bases often have a bitter taste and are found in foods less frequently than acids. A large class of inorganic compounds with. Inorganic Base Name.
From chem.libretexts.org
3.6 Names and Formulas of Compounds Chemistry LibreTexts Inorganic Base Name Bases have properties that mostly contrast with those of acids. a base is a substance that can neutralize the acid by reacting with hydrogen ions. Bases can be either strong or weak, just as acids can. Bases also change the color of. Bases often have a bitter taste and are found in foods less frequently than acids. when. Inorganic Base Name.